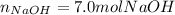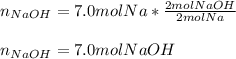Chemistry, 25.05.2021 23:00, yudayang2012pa9u8p
2Na + 2H2O --> 2NaOH + H2 How many moles of NaOH can be produced from 7.0 moles of Na Find theoretical yield in moles PLSS HELP ME SOMEBODY 3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
Do you know the correct answer?
2Na + 2H2O --> 2NaOH + H2 How many moles of NaOH can be produced from 7.0 moles of Na Find theore...
Questions in other subjects:


Mathematics, 30.06.2019 22:00

Mathematics, 30.06.2019 22:00


Spanish, 30.06.2019 22:00

Mathematics, 30.06.2019 22:00


Mathematics, 30.06.2019 22:00