Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
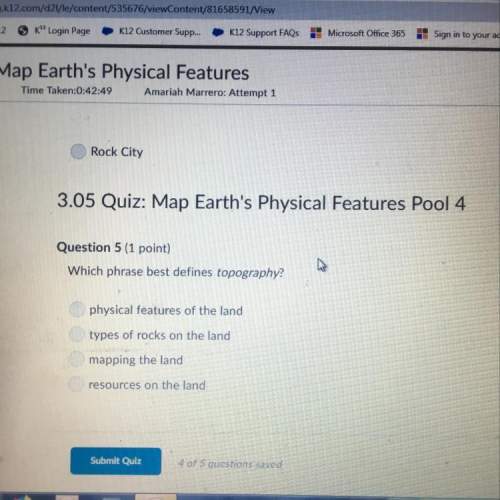
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 02:50, JuniperGalaxy
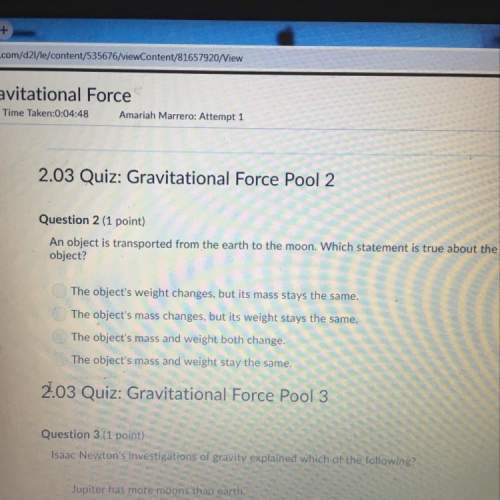
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1
Do you know the correct answer?
In the following reaction, C5H12(1) + 8 O2 (g) - 6 H2O (g) + 5 CO2 (g), how many
moles of water (H2...
Questions in other subjects:






Mathematics, 25.07.2020 20:01