
Chemistry, 25.05.2021 18:40, OkayLearn5522
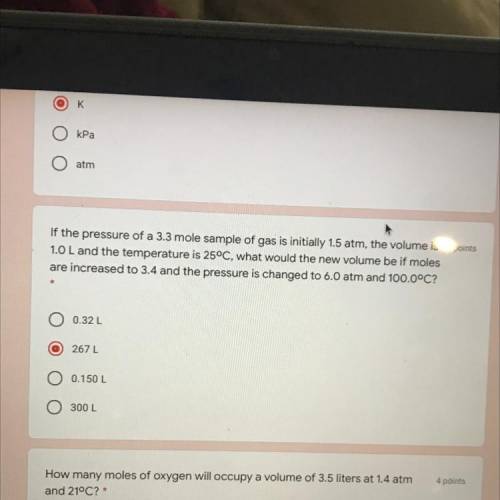
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and the temperature is 25°C, what would the new volume be if moles
are increased to 3.4 and the pressure is changed to 6.0 atm and 100.0°C?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1
Do you know the correct answer?
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and...
Questions in other subjects:


Mathematics, 08.03.2021 23:50


French, 08.03.2021 23:50





Mathematics, 08.03.2021 23:50






