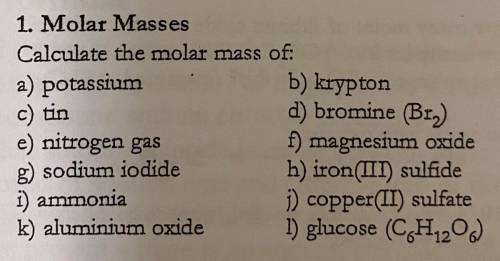Calculate the molar masses of the following:
...

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 10.09.2021 21:40

Mathematics, 10.09.2021 21:40


Mathematics, 10.09.2021 21:40

Mathematics, 10.09.2021 21:40

Mathematics, 10.09.2021 21:40

History, 10.09.2021 21:40



Mathematics, 10.09.2021 21:40