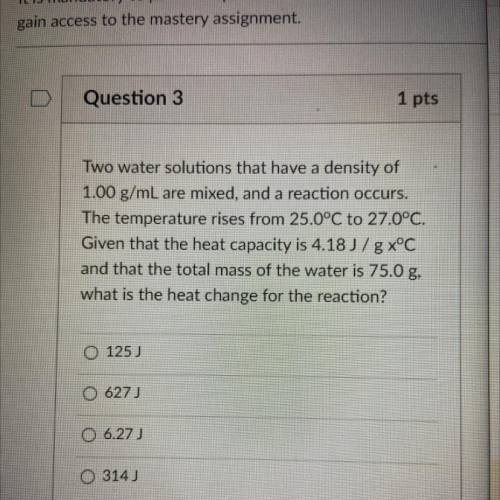
Two water solutions that have a density of
1.00 g/mL are mixed, and a reaction occurs.
The te...

Chemistry, 25.05.2021 08:50, llnapier8924
Two water solutions that have a density of
1.00 g/mL are mixed, and a reaction occurs.
The temperature rises from 25.0°C to 27.0°C.
Given that the heat capacity is 4.18 J/g x°C
and that the total mass of the water is 75.0 g,
what is the heat change for the reaction?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3


Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
Do you know the correct answer?
Questions in other subjects:


English, 16.03.2020 18:23




History, 16.03.2020 18:23

Mathematics, 16.03.2020 18:23

History, 16.03.2020 18:23

Biology, 16.03.2020 18:23

History, 16.03.2020 18:24






