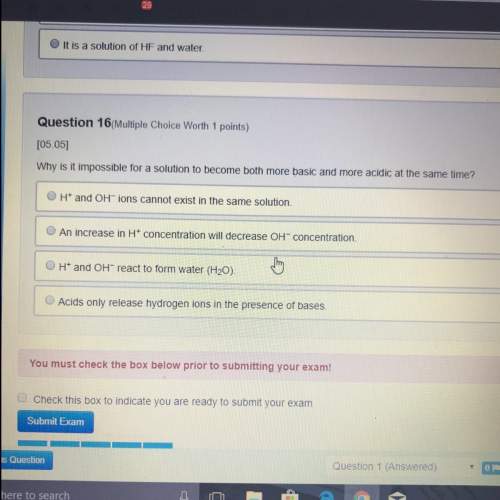
Identify the FALSE statement about orbitals.
o There is one s orbital for each energy
level.<...

Identify the FALSE statement about orbitals.
o There is one s orbital for each energy
level.
0 The symbol 4p' means there are 4
electrons in the 5 p orbitals.
0 They are regions of space likely to be
occupied by electrons around a
nucleus
0 There are four orbitals in the second
energy level
o in each energy level greater than
one, there are three p orbitals

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
Do you know the correct answer?
Questions in other subjects:


History, 10.10.2019 16:50

Chemistry, 10.10.2019 16:50


English, 10.10.2019 16:50



Mathematics, 10.10.2019 16:50