Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, KieraKimball
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1


Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
Do you know the correct answer?
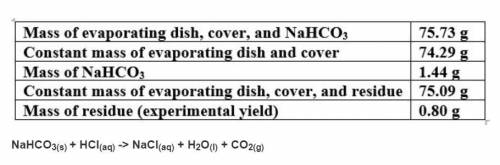
Based on a theoretical yield of 1.00 grams NaCl, calculate the mass in grams of hydrochloric acid yo...
Questions in other subjects:



Biology, 22.05.2020 23:05

Social Studies, 22.05.2020 23:05



History, 22.05.2020 23:05

Mathematics, 22.05.2020 23:05