
Chemistry, 25.05.2021 01:00, gayshipsandcoffee
Mole Conversion -
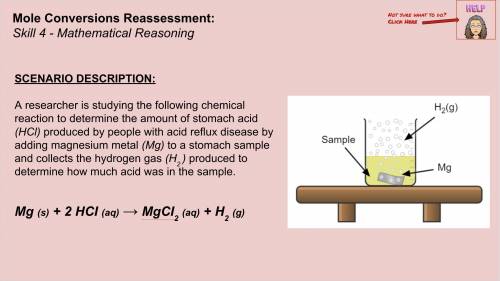
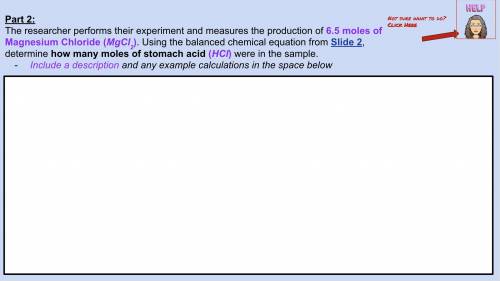
The researcher measures 6.5 moles of Magnesium Chloride (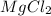 ). Using balanced equation Mg + 2 HCl --->
). Using balanced equation Mg + 2 HCl ---> 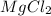 +
+ 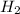 , how many moles of HCl were in the sample?
, how many moles of HCl were in the sample?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1


Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
Do you know the correct answer?
Mole Conversion -
The researcher measures 6.5 moles of Magnesium Chloride (). Using balanced equati...
Questions in other subjects:

Physics, 11.07.2019 00:10














