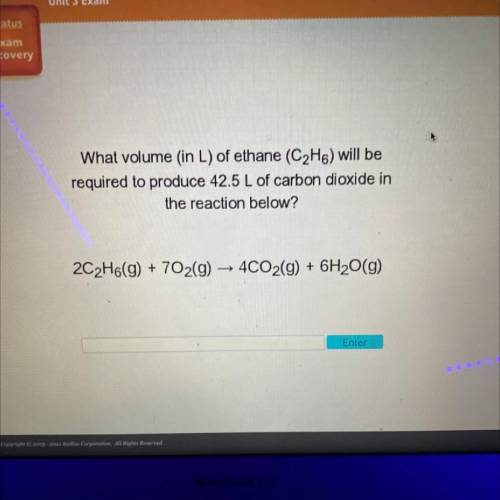
What volume (in L) of ethane (C2H6) will be
required to produce 42.5 L of carbon dioxide in
t...

Chemistry, 25.05.2021 01:00, FavvBella84
What volume (in L) of ethane (C2H6) will be
required to produce 42.5 L of carbon dioxide in
the reaction below?
2C2H6(g) + 702(g) → 4CO2(g) + 6H2O(g)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 05:00, jjoyner
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2


Chemistry, 23.06.2019 09:20, weridness80
Which of the following occurs along coasts during the day?
Answers: 3
Do you know the correct answer?
Questions in other subjects:






Mathematics, 07.07.2019 12:00

History, 07.07.2019 12:00








