
Chemistry, 24.05.2021 21:40, jadejordan8888
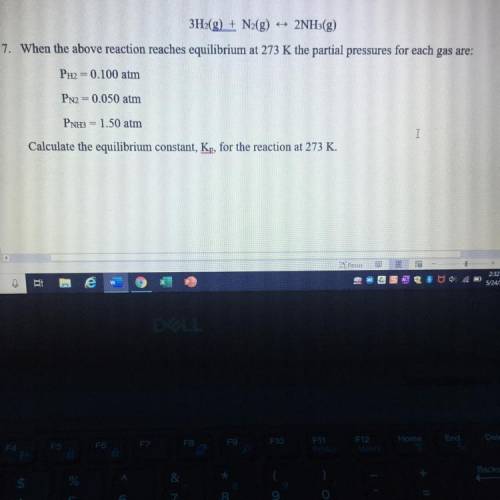
3H2(g) + N2(g) 2NH3(g)
When the above reaction reaches equilibrium at 273 K the partial pressures for each gas are:
PH2 = 0.100 atm
PN2 = 0.050 atm
PNH3 = 1.50 atm
Calculate the equilibrium constant, Kp, for the reaction at 273 K.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 01:30, kaitie60
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
Do you know the correct answer?
3H2(g) + N2(g) 2NH3(g)
When the above reaction reaches equilibrium at 273 K the partial pressures f...
Questions in other subjects:


Mathematics, 22.11.2019 20:31




Biology, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31

History, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31






