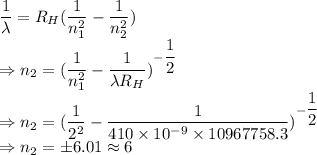Chemistry, 24.05.2021 19:00, aylengarcia090
An electron in an unknown energy level of a hydrogen atom transitions to the n=2 level and emits a photon with wavelength 410 nm in the process. What was the initial energy level? Use R[infinity]=2.179×10−18J for the hydrogen atom Rydberg constant. Use h=6.626×10−34 Js for Planck's constant. Use c=2.998×108ms for the speed of light. Your answer should be a whole number.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
Do you know the correct answer?
An electron in an unknown energy level of a hydrogen atom transitions to the n=2 level and emits a p...
Questions in other subjects:

Mathematics, 11.03.2021 19:40


Mathematics, 11.03.2021 19:40

English, 11.03.2021 19:40

Social Studies, 11.03.2021 19:40



Mathematics, 11.03.2021 19:40


Mathematics, 11.03.2021 19:40


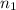 = Final energy level = 2
= Final energy level = 2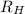 = Rydberg constant =
= Rydberg constant = 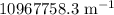
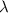 = Wavelength = 410 nm
= Wavelength = 410 nm