
Chemistry, 24.05.2021 18:20, lizbethmillanvazquez
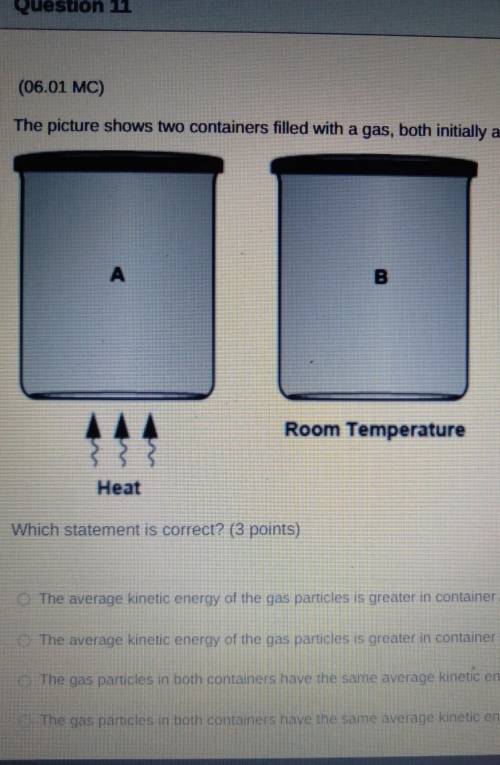
The picture shows two containers filled with a gas, both initially at room temperature.
A Heat
B Room Temperature
Which statement is correct?
The average kinetic energy of the gas particles is greater in container A because its particles move faster.
The average kinetic energy of the gas particles is greater in container B because it has a lower temperature.
The gas particles in both containers have the same average kinetic energy because they have the same volume.
The gas particles in both containers have the same average kinetic energy because they have equal number of particles

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
Do you know the correct answer?
The picture shows two containers filled with a gas, both initially at room temperature.
A Heat
Questions in other subjects:






Advanced Placement (AP), 25.01.2020 23:31



Mathematics, 25.01.2020 23:31

History, 25.01.2020 23:31






