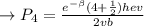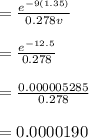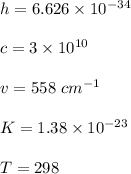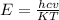Chemistry, 24.05.2021 14:00, romanlittlewood
The first excited vibrational energy level of diatomic chlo- rine (Cl2) is 558 cm^-1 above the ground state. Wave- numbers, the units in which vibrational frequencies are usually recorded, are effectively units of energy, with 1 cm ^-1 = 1.986445 X 10^-23 J. If every vibrational energy level is equally spaced, and has a degeneracy of 1, sum over the lowest 4 vibrational levels to obtain a vibrational partition function for chlorine.
Required:
Determine the populations of each of the four levels at 298 K and the average molar vibrational energy (Em. vib) for chlorine at 298 K.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1


Do you know the correct answer?
The first excited vibrational energy level of diatomic chlo- rine (Cl2) is 558 cm^-1 above the groun...
Questions in other subjects:



Mathematics, 09.12.2021 03:30


Computers and Technology, 09.12.2021 03:30

Social Studies, 09.12.2021 03:30


Mathematics, 09.12.2021 03:30