
Chemistry, 24.05.2021 07:30, lovelife132015
You react hydrogen gas with chlorine gas according to the following reaction:
H2(g) * Cl2(g) -> 2HCl(g)
What mass of HCl(g) can be produced from 2.5 * 10^3 g of hydrogen gas with an excess of chlorine gas?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
Do you know the correct answer?
You react hydrogen gas with chlorine gas according to the following reaction:
H2(g) * Cl2(g) ->...
Questions in other subjects:









Mathematics, 10.06.2021 19:00


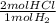 = 2500 mol HCl
= 2500 mol HCl




