
In order to make calcium hydroxide, scientists combine calcium oxide with water
according to the reaction below.
Ca0 + 2H2O -> Ca(OH)2
Scientists believe they will need about 4.1x10^22 g of calcium hydroxide to
neutralize some of the carbonic acid to impact the pH, Determine the mass of
calcium oxide needed to produce 4.1x10^22 g of calcium hydroxide in excess
water.
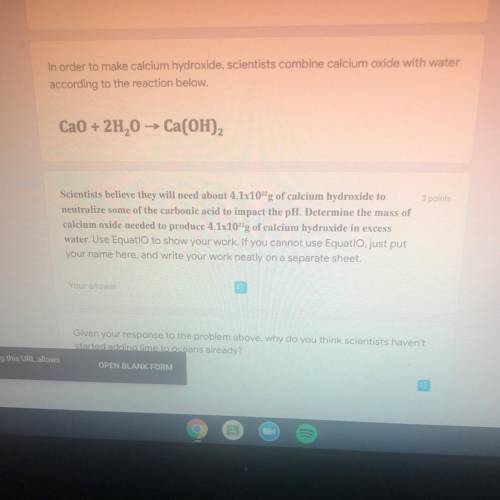

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, maddietomlinson113
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a. inner transition b. noble gases c. representative d. transition
Answers: 2

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
Do you know the correct answer?
In order to make calcium hydroxide, scientists combine calcium oxide with water
according to the re...
Questions in other subjects:

English, 27.09.2019 02:30















