
Chemistry, 23.05.2021 23:20, gracieorman4
The molecules BF3 and NF3 , have similar formulas but different molecular structures. Explain this by determining the molecular structure of each. (HELP ME ASAP PLEASE)
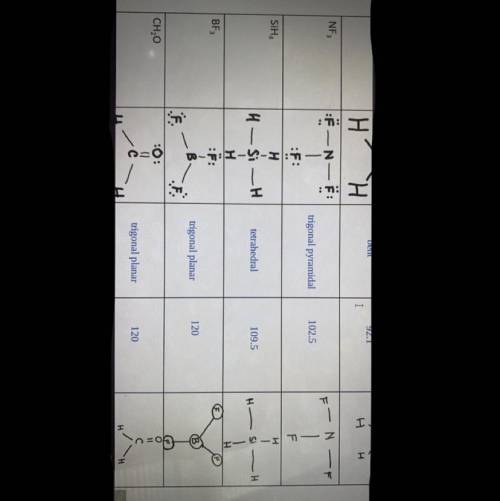

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
Do you know the correct answer?
The molecules BF3 and NF3 , have similar formulas but different molecular structures. Explain this b...
Questions in other subjects:

History, 24.10.2020 07:40



Mathematics, 24.10.2020 07:40




History, 24.10.2020 07:40

Mathematics, 24.10.2020 07:40

Mathematics, 24.10.2020 07:40






