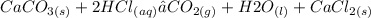Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, hoytkeke6776
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1
Do you know the correct answer?
CaCO3(s) + HCl(aq) -> CO2(g) + H2O(l) + CaCl2(aq)...
Questions in other subjects:

Computers and Technology, 29.08.2019 23:00

Mathematics, 29.08.2019 23:00

Mathematics, 29.08.2019 23:00


Physics, 29.08.2019 23:00

Mathematics, 29.08.2019 23:00



Mathematics, 29.08.2019 23:00

Mathematics, 29.08.2019 23:00