
Chemistry, 21.05.2021 22:30, joelpimentel
A spirit burner used 1.00 g methanol to raise the temperature of 100.0 g water in a metal can from 28.00C to 58.0C. Calculate the heat of combustion in kJ/mol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 23:00, liv467
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 08:00, codybrocs9624
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
Do you know the correct answer?
A spirit burner used 1.00 g methanol to raise the temperature of 100.0 g water in a metal can from 2...
Questions in other subjects:









History, 10.02.2021 21:40


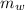 = 100 g
= 100 g




