Answers: 1
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 04:31, laurenbreellamerritt
How big are the bighest ocean waves at mavericks
Answers: 1
Do you know the correct answer?
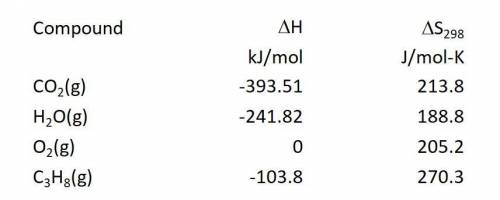
Calculate the entropy change in J/mol-K for the reaction: C3H8(g) + 5O2(g) ---> 3CO2(g) + 4H2O(g)...
Questions in other subjects:




History, 04.02.2020 15:01

English, 04.02.2020 15:01