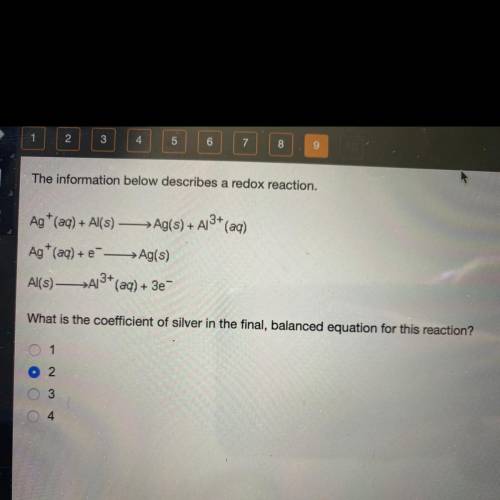
The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (a...

Chemistry, 20.05.2021 22:40, lilpeepxliltracy
The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (aq) + --> Ag(s)
Al(s)—>A13+ (aq) + 3e-
What is the coefficient of silver in the final, balanced equation for this reaction?
1
2
3
4

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jasmineharris121
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
Do you know the correct answer?
Questions in other subjects:










Mathematics, 27.08.2019 19:30






