
Chemistry, 20.05.2021 07:10, lindseylewis313
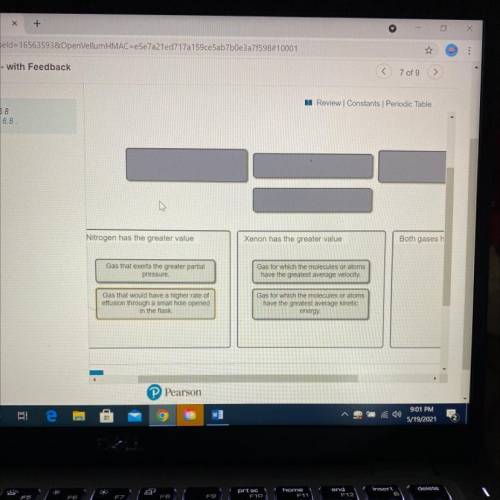
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
Sort the conditions based on the gas described.
Drag the appropriate items to their respective bins.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2



Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
Do you know the correct answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
Sort t...
Questions in other subjects:

History, 27.07.2019 03:00

Business, 27.07.2019 03:00


Mathematics, 27.07.2019 03:00


Mathematics, 27.07.2019 03:00

Mathematics, 27.07.2019 03:00

History, 27.07.2019 03:00


History, 27.07.2019 03:00






