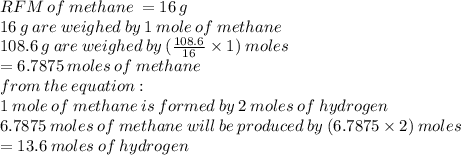Chemistry, 20.05.2021 01:00, solikhalifeoy3j1r
For the reaction C + 2H2 → CH4, how many moles of hydrogen are needed to make 108.6 grams of methane, CH4 ?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
Do you know the correct answer?
For the reaction C + 2H2 → CH4, how many moles of hydrogen are needed to make 108.6 grams of methane...
Questions in other subjects:


Mathematics, 11.03.2021 06:10

Mathematics, 11.03.2021 06:10

Mathematics, 11.03.2021 06:10



History, 11.03.2021 06:10



Mathematics, 11.03.2021 06:10