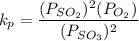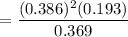Chemistry, 19.05.2021 19:10, sarahaziz9526
A student ran the following reaction in the laboratory at 1080 K: 2SO3(g) 2SO2(g) + O2(g) When she introduced SO3(g) at a pressure of 0.948 atm into a 1.00 L evacuated container, she found the equilibrium partial pressure of SO3(g) to be 0.369 atm. Calculate the equilibrium constant, Kp, she obtained for this reaction. Kp =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
Do you know the correct answer?
A student ran the following reaction in the laboratory at 1080 K: 2SO3(g) 2SO2(g) + O2(g) When she i...
Questions in other subjects:

History, 01.07.2019 22:30


Geography, 01.07.2019 22:30

Mathematics, 01.07.2019 22:30



Mathematics, 01.07.2019 22:30

Mathematics, 01.07.2019 22:30



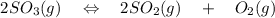
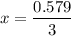
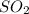 = 0.193 x 2
= 0.193 x 2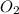 = 0.193 atm
= 0.193 atm