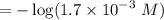Chemistry, 19.05.2021 19:10, shealwaysknows23
Calculate the concentration of H3O+ ions present in a solution of HCl that has a measured pH of 1.510 .
M
HCl, the acid in Part 1, is a strong acid. The concentration of H3O+ ions in this solution is the same as the initial concentration of the acid.
A solution of the weak acid CH3COOH with the same initial concentration of acid will have a pH that is (Higer, lower, the same) the pH of the HCl solution.
Rank the following solutions in order of increasing acidity, placing the most acidic solution at the left. (CH3COOH is approximately 1.0% ionized at this concentration.)
pH= 0.00, pH= 7.45, [HCl]= .15M, [CH3COOH]= .15M
order of acidity
most acidic > least acidic

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, netflixacc0107
Achemist requires 6.00 liters of 0.320 m h2so4 solution. how many grams of h2so4 should the chemist dissolve in water? 121 grams 159 grams 176 grams 188 grams
Answers: 2

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
Do you know the correct answer?
Calculate the concentration of H3O+ ions present in a solution of HCl that has a measured pH of 1.51...
Questions in other subjects:

Biology, 03.02.2021 02:20

Mathematics, 03.02.2021 02:20

Mathematics, 03.02.2021 02:20


Mathematics, 03.02.2021 02:20






![$[H_3O^+]$](/tpl/images/1334/2671/dab07.png) is pH =
is pH = ![$- \log [H_3O^+]$](/tpl/images/1334/2671/b0a97.png)
![$[H_3O^+]=10^{-pH}$](/tpl/images/1334/2671/b447d.png)

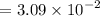
![$=- \log[0.15]$](/tpl/images/1334/2671/efddc.png)
 is as follows :
is as follows :
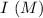 0.15 0 0
0.15 0 0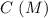 -x +x +x
-x +x +x 0.15 - x x x
0.15 - x x x![$K_a=\frac{[CH_3COO^-][H_3O^+]}{[CH_3COOH]}$](/tpl/images/1334/2671/7fb51.png)

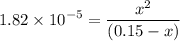




![$[H_3O^+] =x=1.7 \times 10^{-3} \ M$](/tpl/images/1334/2671/663fb.png)