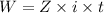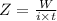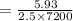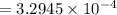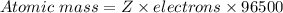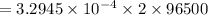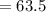A current of 2.50 amps is passed through an electrolytic cell containing a solution of M(NO3)2, where M represents an unidentified metal. The only reaction that occurs at the cathode is the deposition of the unknown metal. After 2.00 hours, 5.93 g of the metal has been deposited.
Required:
Determine the chemical symbol of the metal.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
Do you know the correct answer?
A current of 2.50 amps is passed through an electrolytic cell containing a solution of M(NO3)2, wher...
Questions in other subjects:





Social Studies, 21.04.2020 21:46

Mathematics, 21.04.2020 21:46


Mathematics, 21.04.2020 21:46



 ".
".