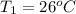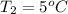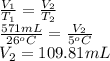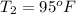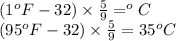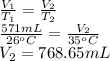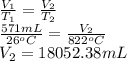Chemistry, 19.05.2021 17:50, naomicervero
A sample of O2 gas occupies a volume of 571 mL at 26 ºC. If pressure remains constant, what would be the new volume if the temperature changed to:
(a) -5 ºC
(b) 95 ºF
(c) 1095 K

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
Do you know the correct answer?
A sample of O2 gas occupies a volume of 571 mL at 26 ºC. If pressure remains constant, what would be...
Questions in other subjects:

Spanish, 30.08.2019 12:50


History, 30.08.2019 12:50

Biology, 30.08.2019 12:50


Social Studies, 30.08.2019 12:50




History, 30.08.2019 12:50


 = 571 mL,
= 571 mL,