2
3 K 4
KM
5
67
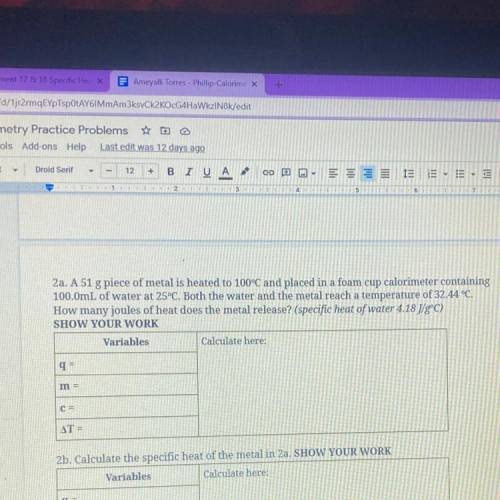
2a. A 51 g piece of metal is heated to 100°C and placed in...

Chemistry, 19.05.2021 01:00, morenoozzie
2
3 K 4
KM
5
67
2a. A 51 g piece of metal is heated to 100°C and placed in a foam cup calorimeter containing
100.0mL of water at 25°C. Both the water and the metal reach a temperature of 32.44 °C.
How many joules of heat does the metal release? (specific heat of water 4.18 J/gºC)
SHOW YOUR WORK
Variables
Calculate here:
q=
m =
C=
AT =

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2


Chemistry, 23.06.2019 03:30, alecnewman2002
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

History, 12.01.2021 22:30

Mathematics, 12.01.2021 22:30

Chemistry, 12.01.2021 22:30

Mathematics, 12.01.2021 22:30


Mathematics, 12.01.2021 22:30

Mathematics, 12.01.2021 22:30


English, 12.01.2021 22:30






