3
1 point
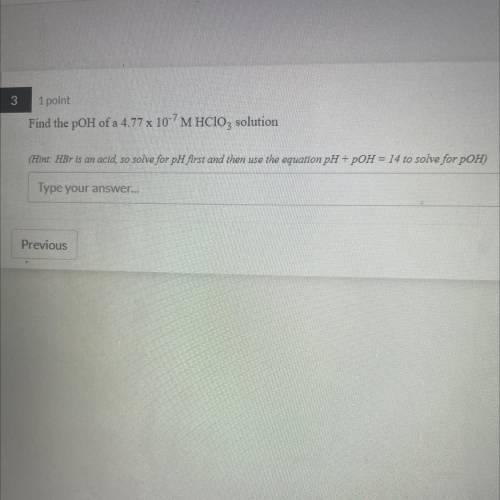
Find the pOH of a 4.77 x 10-7 M HClO3 solution
(Hint: HBr is an acid, so solv...


Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 06:30, shateece
(04.05 hc) analyze the given diagram of the carbon cycle below. an image of carbon cycle is shown. the sun, a cloud, two trees, one on the left and the other on the right, an animal, lake, and a factory are shown in the image. an arrow is shown from the sun towards the left tree marked a. the sun is marked b. there is an arrow from the air above the clouds, marked c, towards the left tree. an arrow from a location close to the ground marked d points towards dead organisms, which is a label under the animal. an arrow marked e points from the right tree straight up to the clouds. an arrow marked f points from the animal straight up to the clouds. an arrow marked g points from the factory towards the air above the clouds, c. there is an arrow pointing from the air to the lake labeled carbonates in water, an arrow pointing down from dead organisms to fossils and fossil fuels, and an arrow from fossils to the factory. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer.
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 04.07.2019 14:30







English, 04.07.2019 14:30