
Chemistry, 18.05.2021 20:30, Nismo3501037
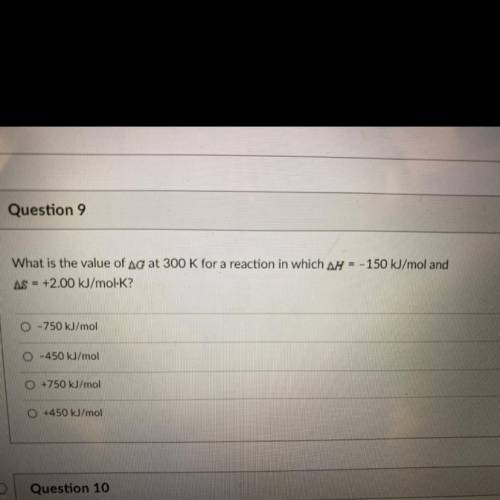
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and AS = +2.00 kJ/mol-K?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 21:20, brendonvernon8
Juju.01) 5 geologic events a group of students designed an experiment in an ice rink to represent the solar system. the steps of the experiment are listed below. 5: geologic events 1. choose a student to represent the sun. the planets are represented by two tennis balls. 2. ask the student to hold the tennis balls in each palm and spin on the ice with arms stretched out. 3. ask the student to draw in the arms after about 10 spins. 4. observe the student's arms rotate faster when they are closer to the body. 05 geologic events enors) the experiment most likely demonstrates that (2 points) 07 discussion-based sessment/module planets exert gravitational force on the sun the sun exerts gravitational force on the planets 3.07 discussion-based ssessment speed of a planet depends on its distance from the sun new version available! (3.0.119) get it now submit 18.07: module exam description
Answers: 3

Chemistry, 23.06.2019 05:10, citlalli30
Name a brittle metal , which is used to galvanize iron
Answers: 1
Do you know the correct answer?
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and
AS = +2.00 kJ/mol-K?...
Questions in other subjects:

Mathematics, 27.01.2021 18:20


Mathematics, 27.01.2021 18:20

Chemistry, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20

Computers and Technology, 27.01.2021 18:20


Mathematics, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20








