
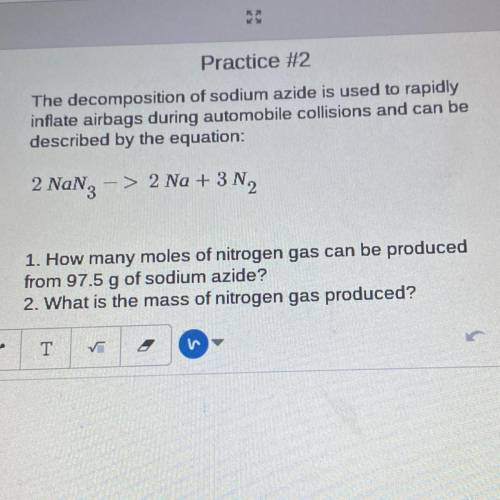
The decomposition of sodium azide is used to rapidly
inflate airbags during automobile collisions and can be
described by the equation:
2 Nang
-> 2 Na + 3N2
1. How many moles of nitrogen gas can be produced
from 97.5 g of sodium azide?
2. What is the mass of nitrogen gas produced?
T
2 X

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1



Do you know the correct answer?
The decomposition of sodium azide is used to rapidly
inflate airbags during automobile collisions a...
Questions in other subjects:

Health, 14.07.2019 01:40







Mathematics, 14.07.2019 01:50


Physics, 14.07.2019 01:50






