
Chemistry, 18.05.2021 19:30, kmontello5
Equal molar quantities of Ca2 and EDTA (H4Y) are added to make a 0.010 M solution of CaY2- at pH 10. The formation constant for CaY2- is 5.0 x 1010 and the fraction of unprotonated EDTA (Y4-) is 0.35 at pH10. Calculate the concentration of free Ca2 in this solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, KeiraDawn
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 21.06.2019 17:30, brasherfamily14
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1
Do you know the correct answer?
Equal molar quantities of Ca2 and EDTA (H4Y) are added to make a 0.010 M solution of CaY2- at pH 10....
Questions in other subjects:

Mathematics, 20.09.2020 01:01


Geography, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01




Mathematics, 20.09.2020 01:01


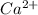 +
+ 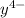 ⇄
⇄ 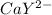
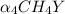 ; ∝₄ = 0.35
; ∝₄ = 0.35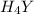 ⇄
⇄ 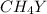 =
= 



