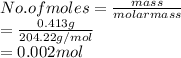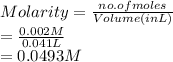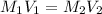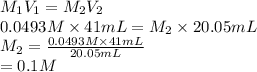Chemistry, 18.05.2021 19:20, christinamonte122
413 mg of dried KHP is dissolved in 41 mL of distilled water and titrated with potassium hydroxide (KOH). If it took 20.05 mL of KOH to reach the endpoint, determine the concentration of KOH. Show calculations.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
Do you know the correct answer?
413 mg of dried KHP is dissolved in 41 mL of distilled water and titrated with potassium hydroxide (...
Questions in other subjects:

Spanish, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20

Business, 20.01.2021 22:20

Arts, 20.01.2021 22:20

Physics, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20



Mathematics, 20.01.2021 22:20