Chemistry, 18.05.2021 18:30, golderhadashaowtatz
Calculate the specific heat capacity of the unknown metal given the
following information; the mass of the metal is 225 g, the temperature
raises by 13C and the energy absorbed was 1363 joules.
A. 0.466 j/gC
B.1.02j/gC
C.0.96 j/gC
D.2.14 j/gC

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, alexisgoss8091
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 00:40, petriajack8375
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
Do you know the correct answer?
Calculate the specific heat capacity of the unknown metal given the
following information; the mass...
Questions in other subjects:

Health, 18.11.2019 05:31


History, 18.11.2019 05:31

Biology, 18.11.2019 05:31




Mathematics, 18.11.2019 05:31



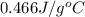 .
.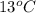
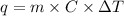
 = change in temperature
= change in temperature