
Chemistry, 18.05.2021 17:40, aahneise02
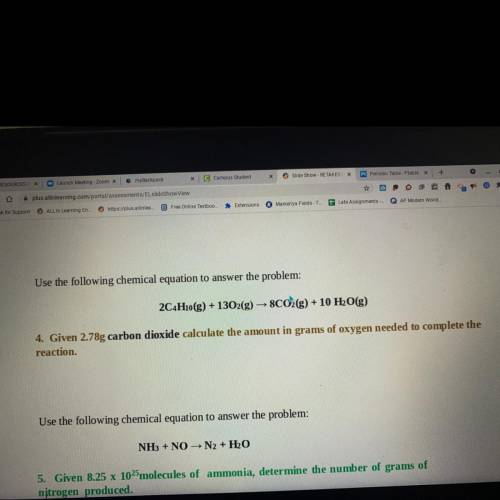
Use the following chemical equation to answer the problem:
2C4H10(g) + 1302(g) → 8CO2(g) + 10 H2O(g)
4. Given 2.78g carbon dioxide calculate the amount in grams of oxygen needed to complete the
reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
Do you know the correct answer?
Use the following chemical equation to answer the problem:
2C4H10(g) + 1302(g) → 8CO2(g) + 10 H2O(g...
Questions in other subjects:

History, 11.03.2021 01:30

History, 11.03.2021 01:30

Mathematics, 11.03.2021 01:30

Physics, 11.03.2021 01:30


Mathematics, 11.03.2021 01:30




Mathematics, 11.03.2021 01:30






