
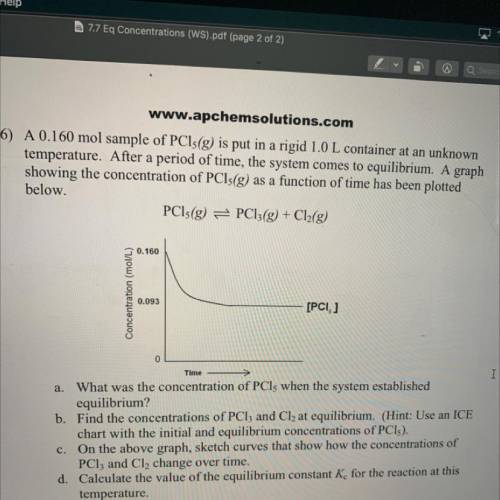
6) A 0.160 mol sample of PCls(g) is put in a rigid 1.0 L container at an unknown
temperature. After a period of time, the system comes to equilibrium. A graph
showing the concentration of PCls(g) as a function of time has been plotted
below.
PCls(g) = PC13(g) + Cl2(g)
0.160
Concentration (mol/L)
0.093
[PCI, ]
I
Time
a. What was the concentration of PCls when the system established
equilibrium?
b. Find the concentrations of PCl3 and Cl2 at equilibrium. (Hint: Use an ICE
chart with the initial and equilibrium concentrations of PCI).
c. On the above graph, sketch curves that show how the concentrations of
PClz and Cl, change over time.
d. Calculate the value of the equilibrium constant K for the reaction at this
temperature.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2


Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
Do you know the correct answer?
6) A 0.160 mol sample of PCls(g) is put in a rigid 1.0 L container at an unknown
temperature. After...
Questions in other subjects:

Mathematics, 20.05.2020 19:58



Mathematics, 20.05.2020 19:59

Mathematics, 20.05.2020 19:59





Mathematics, 20.05.2020 19:59






