
Chemistry, 18.05.2021 07:50, zuleromanos
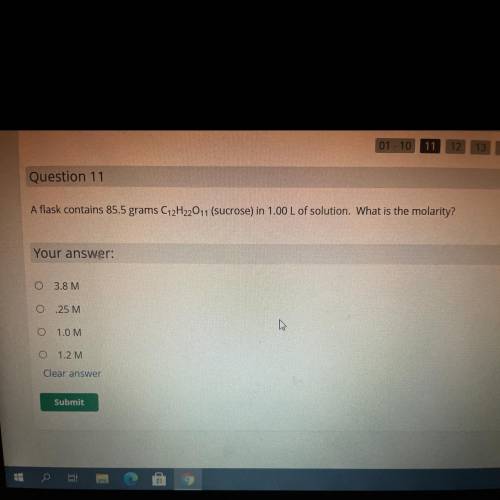
A flask contains 85.5 grams C12H2011 (sucrose) in 1.00 L of solution. What is the molarit
Your answer.
3.8 M
25 M
10M
1.2M

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
Do you know the correct answer?
A flask contains 85.5 grams C12H2011 (sucrose) in 1.00 L of solution. What is the molarit
Your answ...
Questions in other subjects:

Spanish, 01.09.2019 09:10

Biology, 01.09.2019 09:10



Business, 01.09.2019 09:10


Physics, 01.09.2019 09:10

History, 01.09.2019 09:10

Arts, 01.09.2019 09:10

Biology, 01.09.2019 09:10






