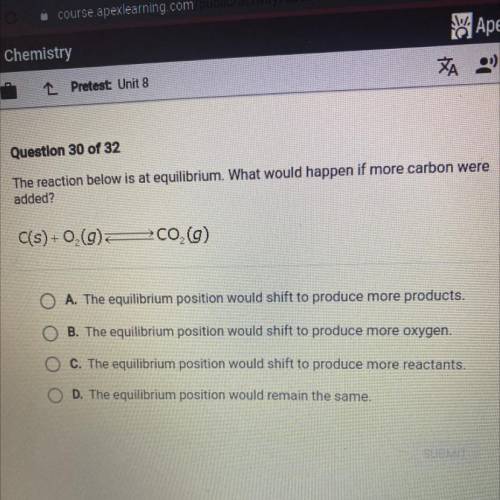
The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9...

The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9)
700 (9)
A. The equilibrium position would shift to produce more products.
B. The equilibrium position would shift to produce more oxygen.
C. The equilibrium position would shift to produce more reactants.
D. The equilibrium position would remain the same.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1


Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 13.10.2020 01:01



Mathematics, 13.10.2020 01:01




Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01






