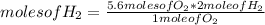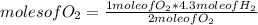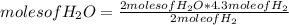Chemistry, 17.05.2021 16:50, genyjoannerubiera
How many moles of water can be produced with 4.3 moles of H2 and 5.6 moles of O2? Which reactant is limiting? How many moles of the excess reactant will be left after the reaction? 2 H2 + O2 2 H2O

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2
Do you know the correct answer?
How many moles of water can be produced with 4.3 moles of H2 and 5.6 moles of O2? Which reactant is...
Questions in other subjects:

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01

Mathematics, 11.09.2020 07:01