

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1


Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2
Do you know the correct answer?
a sample of sulfur contains sulfur-32, sulfur-33, sulfur-34, and sulfur-36 atoms. why these atoms ca...
Questions in other subjects:



Geography, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01



Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


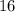 .) Hence, the atomic number of each atom would be equal to the atomic number of sulfur:
.) Hence, the atomic number of each atom would be equal to the atomic number of sulfur: 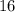 .
.  belongs to argon.
belongs to argon. (mass number
(mass number 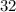 ) contains
) contains  neutrons, whereas sulfur-
neutrons, whereas sulfur-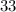 (mass number
(mass number 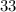 ) contains
) contains  neutrons. (The
neutrons. (The 



