
Chemistry, 17.05.2021 09:40, xxtonixwilsonxx
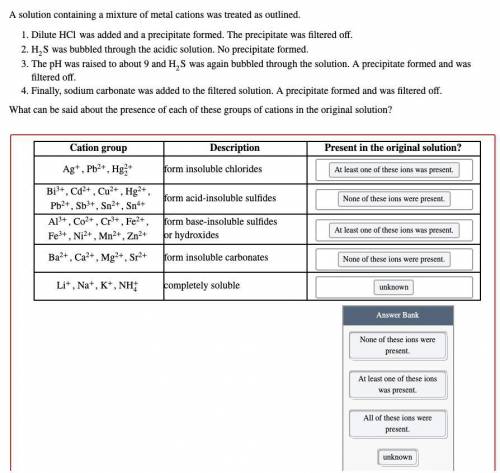
A solution containing a mixture of metal cations was treated as outlined.
Dilute HCl was added and a precipitate formed. The precipitate was filtered off.
H2S was bubbled through the acidic solution. No precipitate formed.
The pH was raised to about 9 and H2S was again bubbled through the solution. A precipitate formed and was filtered off.
Finally, sodium carbonate was added to the filtered solution. A precipitate formed and was filtered off.
What can be said about the presence of each of these groups of cations in the original solution?
Options: None of these ions were present
At least one of these ions was present
All of these ions were present
Unknown

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2


Chemistry, 23.06.2019 20:00, FailingstudentXD
State and explain the three laws of chemical combination
Answers: 1
Do you know the correct answer?
A solution containing a mixture of metal cations was treated as outlined.
Dilute HCl was added and...
Questions in other subjects:







Mathematics, 29.02.2020 00:24

Mathematics, 29.02.2020 00:24







