
Chemistry, 16.05.2021 01:30, carlshiabrown
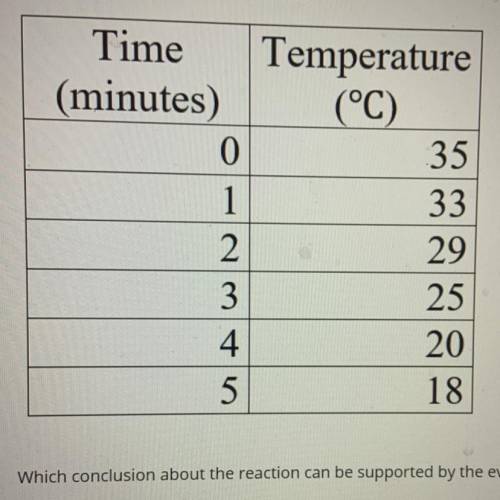
(12 points multiple choice )A chemist pours two chemicals into a beaker and observes their reactions. Using the data table determine if the reaction is
a. Exothermic and spontaneous
b. Endothermic and spontaneous
c. Exothermic and not spontaneous

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 23.06.2019 11:00, lildestinyquintana
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2

Chemistry, 23.06.2019 13:20, TayJoker966
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2
Do you know the correct answer?
(12 points multiple choice )A chemist pours two chemicals into a beaker and observes their reactions...
Questions in other subjects:


Computers and Technology, 28.09.2020 20:01

Mathematics, 28.09.2020 20:01

Business, 28.09.2020 20:01

Mathematics, 28.09.2020 20:01


Mathematics, 28.09.2020 20:01


English, 28.09.2020 20:01






