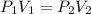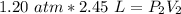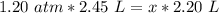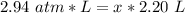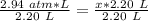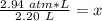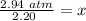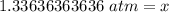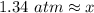Chemistry, 15.05.2021 06:20, shayshay7874
6. Calculate the new pressure in atm if 2.45 L of a gas at a pressure of 1.20 atm is contracted to a new volume of 2.20 L

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1


Chemistry, 23.06.2019 12:00, angieplasencia8
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
Do you know the correct answer?
6. Calculate the new pressure in atm if 2.45 L of a gas at a pressure of 1.20 atm is contracted to a...
Questions in other subjects:




Social Studies, 19.08.2019 07:30

Geography, 19.08.2019 07:30


Social Studies, 19.08.2019 07:30

Mathematics, 19.08.2019 07:30


Mathematics, 19.08.2019 07:30