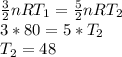Heat is added to two identical samples of a monatomic ideal gas. In the first sample, the heat is added while the volume of the gas is kept constant, and the heat causes the temperature to rise by 80 K. In the second sample, an identical amount of heat is added while the pressure (but not the volume) of the gas is kept constant. By how much does the temperature of this sample increase

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Do you know the correct answer?
Heat is added to two identical samples of a monatomic ideal gas. In the first sample, the heat is ad...
Questions in other subjects:


Health, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Health, 18.03.2021 01:30

Social Studies, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Chemistry, 18.03.2021 01:30