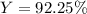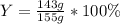Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2


Chemistry, 22.06.2019 19:20, Lovelybunny321
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
Do you know the correct answer?
For the reaction 2Na + Cl2 2NaCl, calculate the percent yield if
155.0 g of chlorine (Cl2) should b...
Questions in other subjects:

Mathematics, 04.06.2021 16:20


English, 04.06.2021 16:20



Mathematics, 04.06.2021 16:20


Mathematics, 04.06.2021 16:20

Mathematics, 04.06.2021 16:20