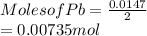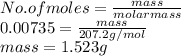When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate PbSO4 is reduced to lead at the cathode and oxidized to solid lead(II) oxide PbO at the anode. Suppose a current of 62.0 is fed into a car battery for 23.0 seconds. Calculate the mass of lead deposited on the cathode of the battery. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
Do you know the correct answer?
When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic...
Questions in other subjects:

History, 17.09.2021 09:00

Mathematics, 17.09.2021 09:00

Geography, 17.09.2021 09:00

Mathematics, 17.09.2021 09:00


History, 17.09.2021 09:00

Physics, 17.09.2021 09:00


History, 17.09.2021 09:00

English, 17.09.2021 09:00


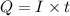
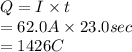
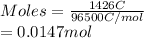
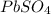 is 2. So, moles deposited by Pb is as follows.
is 2. So, moles deposited by Pb is as follows.