
Chemistry, 15.05.2021 03:10, tammycute01
He calculated that he should produced 6.0 grams of Mg2N2 . He actually produced 4.8 grams of Mg3N2 . What is the percent yield ?
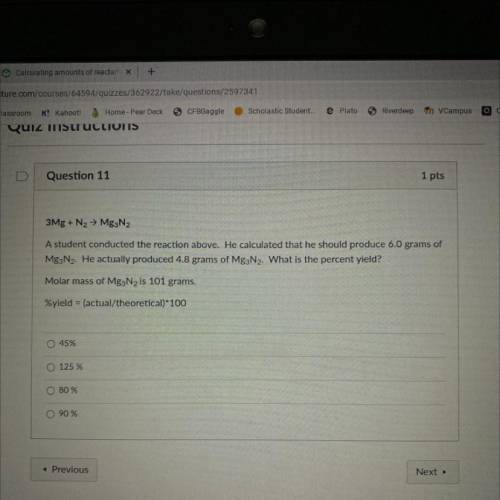

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1
Do you know the correct answer?
He calculated that he should produced 6.0 grams of Mg2N2 . He actually produced 4.8 grams of Mg3N2 ....
Questions in other subjects:

Mathematics, 18.02.2021 18:10

Mathematics, 18.02.2021 18:10

Biology, 18.02.2021 18:10

Physics, 18.02.2021 18:10


Biology, 18.02.2021 18:10

English, 18.02.2021 18:10








