Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
Do you know the correct answer?
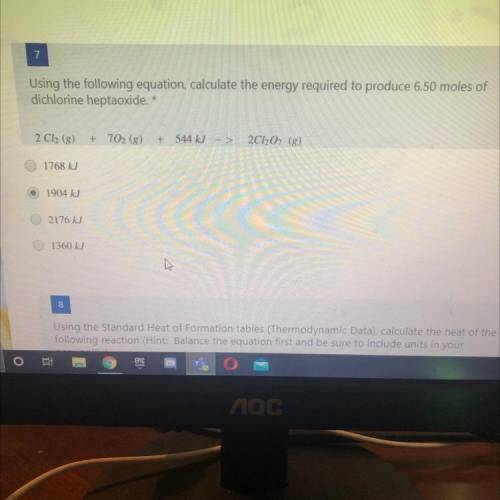
Using the following equation, calculate the energy required to produce 6.50 moles of
dichlorine hep...
Questions in other subjects:

Mathematics, 20.11.2020 04:10


Computers and Technology, 20.11.2020 04:10

Mathematics, 20.11.2020 04:10


Biology, 20.11.2020 04:10

Mathematics, 20.11.2020 04:10



Biology, 20.11.2020 04:10