
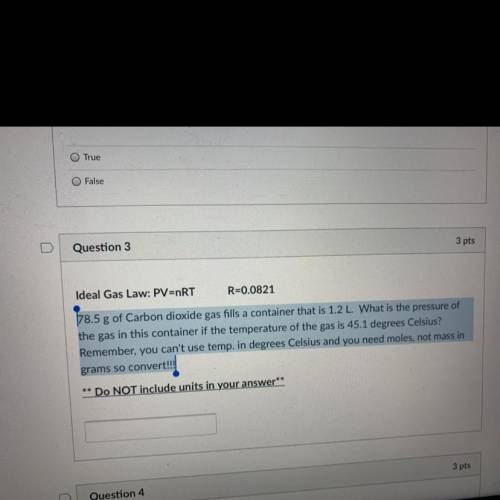
PLEASSE HELP! 78.5 g of Carbon dioxide gas fills a container that is 1.2 L. What is the pressure of the gas in this container if the temperature of the gas is 45.1 degrees Celsius? Remember, you can't use temp. in degrees Celsius and you need moles, not mass in grams so convert!!!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
Do you know the correct answer?
PLEASSE HELP!
78.5 g of Carbon dioxide gas fills a container that is 1.2 L. What is the pressure of...
Questions in other subjects:

Social Studies, 31.05.2020 04:59








Mathematics, 31.05.2020 04:59






