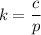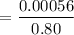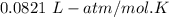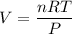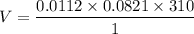The solubility of N2in blood at 37°C and at a partial pressure of 0.80 atm is 5.6 × 10−4mol/L. A deep-sea diver breathes compressed air with the partial pressure of N2equal to 4.0 atm. Assume that the total volume of blood in the body is 5.0 L. Calculate the amount of N2gas released (in liters at 37°C and 1 atm) when the diver returns to the surface of the water, where the partial pressure ofN2is 0.80 atm.

Answers: 1
Other questions on the subject: Chemistry




Do you know the correct answer?
The solubility of N2in blood at 37°C and at a partial pressure of 0.80 atm is 5.6 × 10−4mol/L. A dee...
Questions in other subjects:

Mathematics, 14.12.2020 03:40

Mathematics, 14.12.2020 03:40

Mathematics, 14.12.2020 03:40

Law, 14.12.2020 03:40

English, 14.12.2020 03:40



Mathematics, 14.12.2020 03:40

Mathematics, 14.12.2020 03:40

SAT, 14.12.2020 03:40