ALL In Learning
Show Summary
Previous
Next
→
CHEM QUIZ 4 7 ON 5 14 21
...

Chemistry, 14.05.2021 18:00, shartman22
ALL In Learning
Show Summary
Previous
Next
→
CHEM QUIZ 4 7 ON 5 14 21
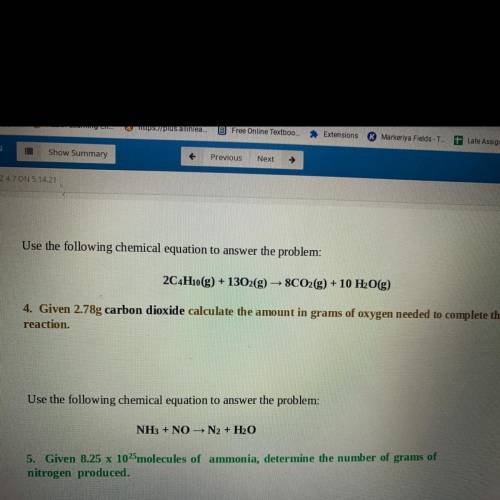
Use the following chemical equation to answer the problem:
2C4H10(g) + 1302(g) → 8CO2(g) + 10 H2O(g)
4. Given 2.78g carbon dioxide calculate the amount in grams of oxygen needed to complete the
reaction.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 14:30, Knownothing
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1

Do you know the correct answer?
Questions in other subjects:



Mathematics, 01.07.2021 15:50





Mathematics, 01.07.2021 15:50







